Comparative Analysis
Compare corynantheidine with major kratom alkaloids—mitragynine, 7-hydroxymitragynine, paynantheine, speciociliatine, and speciogynine—using validated occurrence data and peer-reviewed receptor/pharmacology results. Figures and download links are embedded.
Data Sources Used
- Occurrence/quantitation (plant & products): validated UPLC–MS/MS ten-alkaloid method; ranges across leaves, extracts, teas, finished products [1].
- US product survey (N≈341; 10 alkaloids quantified): mean levels across finished products; screen against non-alkaloid adulterants [2].
- Chemotypes/variability (UPLC-HRMS across plants & products): distinct alkaloid profiles (affects minor-alkaloid levels) [3].
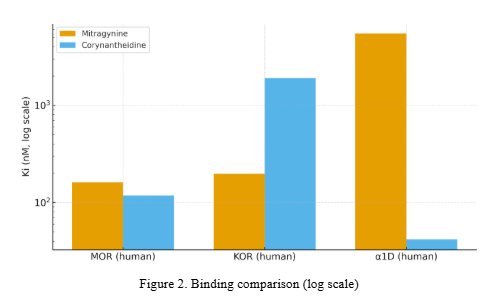
- Receptor binding/function: human MOR/KOR and α1D binding; MOR signaling and β-arrestin; in vivo antinociception [4].
- Serotonin receptor data (paynantheine comparator): 5-HT₁A activity [5].
- Potent MOR comparator (7-hydroxymitragynine): antinociception and context [6].