Paynantheine and the Opioid Receptor System
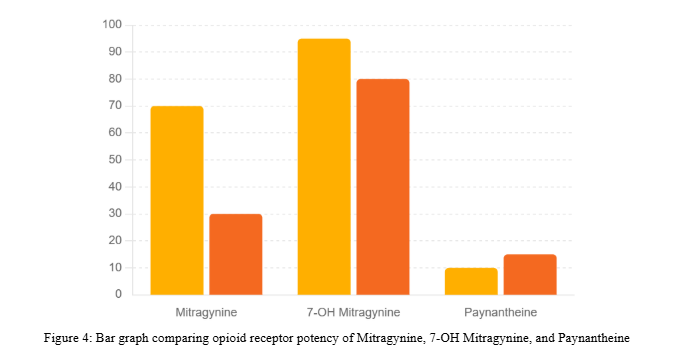
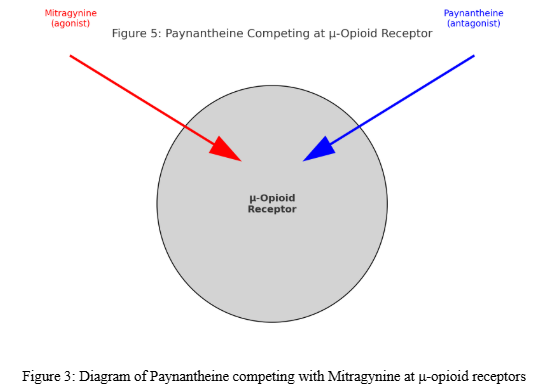
While Mitragynine and 7-Hydroxymitragynine are known opioid receptor agonists, Paynantheine shows antagonistic activity at opioid receptors. Its weaker receptor interactions highlight its distinct pharmacological contribution compared to kratom’s primary alkaloids.
Binding Affinity at Opioid Receptors
Studies using radioligand binding assays demonstrate that Paynantheine exhibits measurable but modest interactions with opioid receptors:
Paynantheine and the Opioid Receptor System
While Mitragynine and 7-Hydroxymitragynine are known opioid receptor agonists, Paynantheine shows antagonistic activity at opioid receptors. Its weaker receptor interactions highlight its distinct pharmacological contribution compared to kratom’s primary alkaloids.
Binding Affinity at Opioid Receptors
- μ-Opioid Receptor (MOR): Competitive antagonist; prevents agonist activity of Mitragynine and 7-OH — PMC, Pharmacological Characterization [1].
- κ-Opioid Receptor (KOR): Competitive antagonist; reduces KOR agonist activity — PMC, Binding Assays [2].
- δ-Opioid Receptor (DOR): Weak / negligible binding; minimal pharmacological impact — PubMed, Receptor Affinity [3].