Corynantheidine : Occurence And Measurement
Occurrence & Quantification
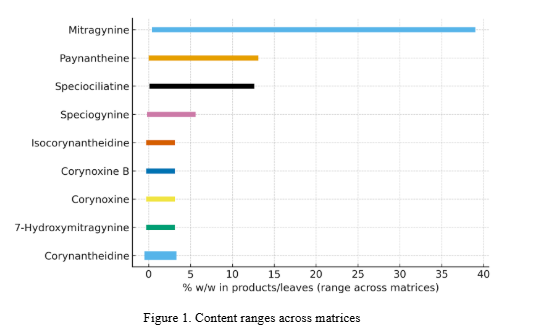
Occurrence (concentration ranges) and quantification methods for corynantheidine in Mitragyna speciosa (kratom) leaves, extracts, and commercial products. Includes validated LC–MS/MS methods, detection/quantitation limits, and product-level surveys. No pharmacology here.
Primary Sources
Primary sources are method papers and analytical surveys with explicit numeric data and validation details. Key inclusions:
- Sharma et al. (2019) – ten-alkaloid UPLC–MS/MS plant/product QC
- King et al. (2020) – single-analyte plasma method
- Kamble et al. (2021) – 11-analyte plasma method (kratom tea & liquid shot)
- Manwill et al. (2022) – plant/product chemotype characterization (UPLC-HRMS)
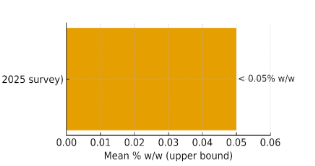
- Large US product survey (2025) – quantified alkaloid content [1]