Toxicology and Safety Assessment of Paynantheine
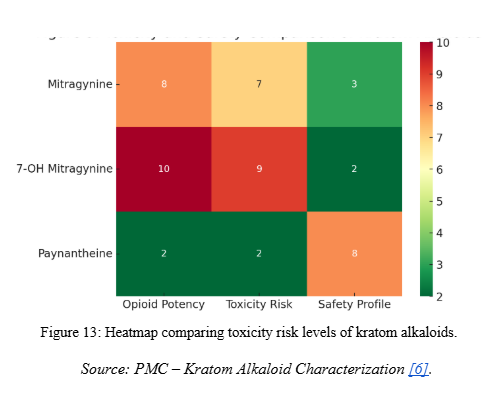
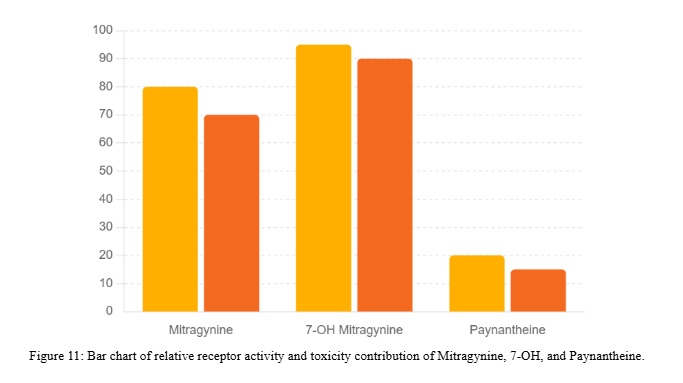
- General observation: Compared to Mitragynine and 7-Hydroxymitragynine, Paynantheine has been less studied for toxicological properties but does not appear to be a major driver of toxicity.
- Animal studies: Detected in pharmacological assays, but no lethal dose (LD₅₀) established specifically for Paynantheine. Most studies assess kratom extracts rather than the isolated alkaloid.
- Relative toxicity: Weaker CNS activity than Mitragynine and 7-OH Mitragynine, suggesting a lower toxicological burden [1].