Pharmacological Profile Of Paynantheine
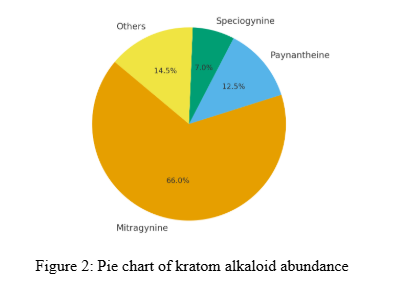
Paynantheine [1] is among the most studied “minor” Kratom alkaloids due to its relatively high abundance and distinct receptor activity. Unlike Mitragynine or 7-Hydroxymitragynine, which act as opioid receptor agonists, Paynantheine demonstrates a unique profile combining opioid receptor antagonism with serotonin receptor modulation. This section explores its chemical identity, occurrence, analytical detection, and receptor binding data, providing a foundation for understanding its pharmacological role.
Chemical Identity & Structure
- Alkaloid class: Paynantheine is classified as an indole alkaloid found in Mitragyna speciosa, ACS Journal of Natural Products [2].
- Molecular formula: C₂₃H₂₈N₂O₅ [3], with a molecular weight of ~408.48 g/mol.
- Structural features: Contains a core indole framework and methoxy substitutions, contributing to receptor-binding properties. ResearchGate – Chemical Structures of Kratom Alkaloids.
Together, these properties position Paynantheine as a chemically distinct alkaloid with potential pharmacological relevance.