At a glance
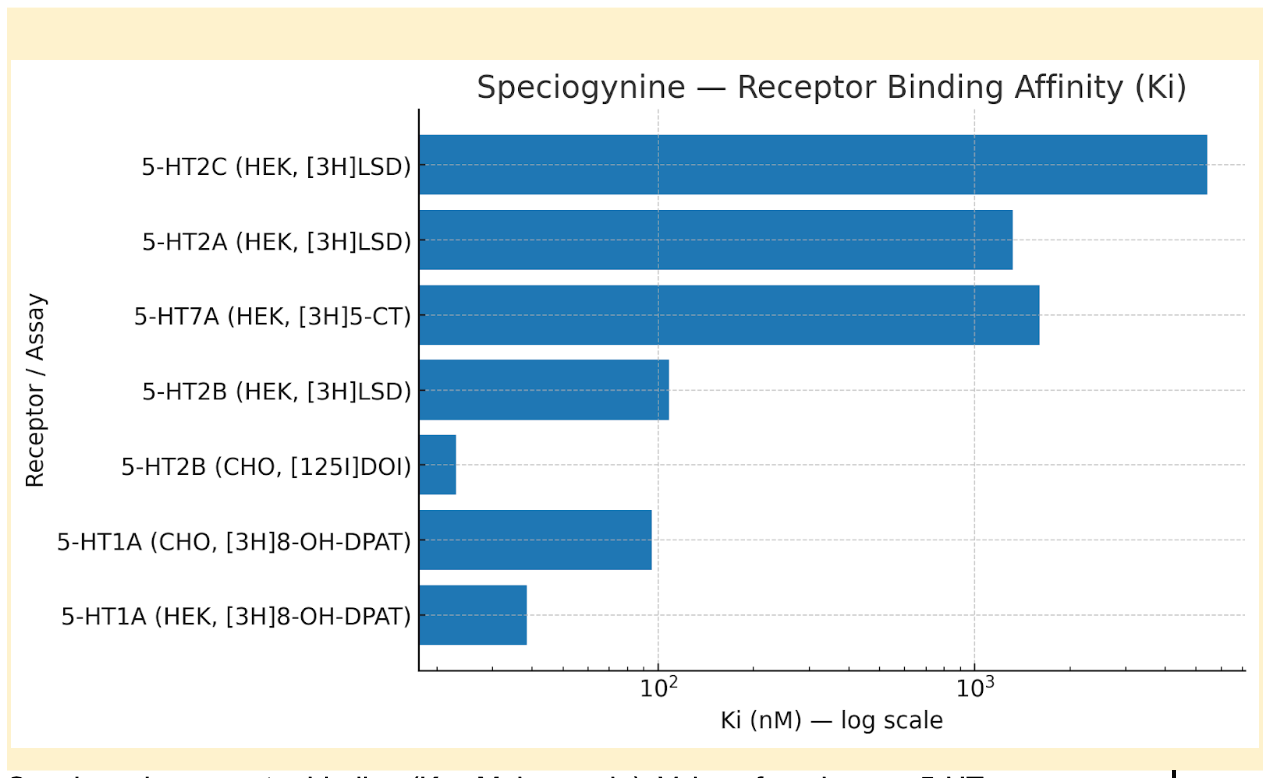
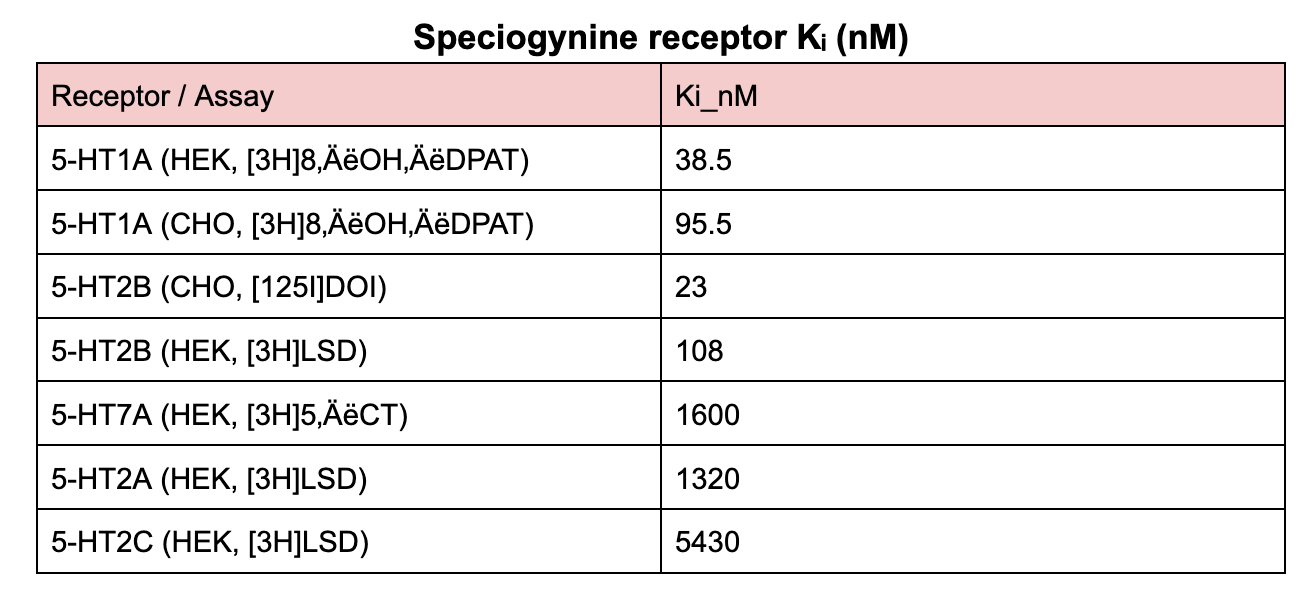
Speciogynine is a natural diastereomer of mitragynine found in Mitragyna speciosa (kratom). The single change at C-20 (20R vs 20S) shifts target engagement: speciogynine shows high-affinity binding at 5-HT₁A and 5-HT₂B but lacks the μ-opioid agonism associated with mitragynine.

| Target | What’s reported |
| 5-HT₁A | High-affinity binding; parent speciogynine shows no agonism in vitro; its 9-O-desmethyl metabolite is a 5-HT₁A agonist. |
| 5-HT₂B | High-affinity binding; parent shows no agonism (inverse partial activity only). |
| Opioid (MOR/KOR/DOR) | Speciogynine is not a MOR agonist under the same assay conditions tested for kratom alkaloids. |

Your cart
Your cart is empty
Recently viewed products